Each ingredient in a recipe serves a purpose. Whether the ingredient is to sweeten, add spice, or thicken, the reaction occurring plays a key role in how the dish turns out.
———————————————————————————————————————————

The Spruce Eats / Diana Chistruga
Natilla Colombiana is a rich, sweet custard dessert that is traditionally served during Christmas gatherings and is greatly enjoyed with a fluffy, crispy, and savory buñuelo (cheese fritter). The main component that makes natilla that thick, rich custard is… you guessed it, cornstarch! This white corn powder is beloved in the kitchen and is a commonly used food thickener.
Cornstarch is used to make gravies, soups, marinades, and curries. In any instance where cornstarch is used as a thickener, it is mixed with water (or liquid of use) first to make a slurry and then added to allow thickening. When mixing cornstarch in cold water (or in natillas case, milk) it doesn’t thicken when the cornstarch comes into contact with the water. It creates a suspension; Why? It only starts to thicken when heat is added. That is thanks to the amylopectin and amylose. To know more about why cornstarch (and the other ingredients of Natilla) act the way they do and how they come together to create Natilla, keep reading.
Click here for a summary of the process of cornstarch.
Original Recipe
Below is the original recipe I chose to investigate and the process.
- 4 ½ cups (1080 ml or a little bit more than a liter) of milk (divided)
- ¼ cup (50 g) white sugar
- ½ cup (107 g) packed brown sugar
- 1 dash ground cloves (optional)
- 4 cinnamon sticks
- ¾ cup (45 g) shredded coconut (optional), I use sweetened shredded coconut
- 1 cup (120 g) cornstarch
- 1 tbs cinnamon powder (for decoration)
- Pour 3½ cups (840 ml) of milk into a big pot. Then add white sugar, brown sugar, cloves, and cinnamon sticks. Stir all ingredients with a wooden spoon and bring milk to a boil over medium-low heat.
- As soon as the milk comes to a boil, remove it from the stove and let it rest for about 5 minutes.
- In the meantime, mix the cornstarch with the remaining cup (240 ml) of milk until it completely dissolves.
- Once the 5 minutes have passed, put the pot back on the stove over medium-low heat. Remove the cinnamon sticks and add the shredded coconut. Then pour the dissolved cornstarch into the hot milk. Stir constantly with a wooden spoon until it thickens and you can see the bottom of the pot.
- Pour immediately into a serving dish or casserole and let it cool for at least an hour. Decorate with the cinnamon powder.
For convenience, a time and activity chart is available to print!
The Magic
Whether a recipe has 2 or 20 ingredients, each one plays a specific role; like a part in a movie.
Milk
Milk is the first and base ingredient of the recipe. It is the dispersed phase of this dish; everything is added to create the gelatinous dessert. It is a complex mixture made up of water, fat, protein (casein and whey), and lactose (milk sugar), with water being the major component at 87-88%. This provides the moisture the cornstarch molecules need in order to gelatinize. Fat globules are dispersed in the water in the milk classifying it as an emulsion. As you boil milk, the components start to separate.
The protein casein is what makes up 80% of milk’s proteins. The proteins, once separated, go through denaturation and start to interact with the small amount of acid found in the brown sugar because of the molasses. Casein proteins themselves don’t have any disulfide bonds to create tertiary structures, making it hard for them to stack onto one another. Once the acid and heat help to open them up, they are able to spread out to form links with other proteins nearby. They then coagulate once the dish is cooled creating a sort of mesh structure to form a gel.
White Sugar
1
White sugar is next. The white sugar serves as a sweetener, containing almost 100% sucrose. As the sugar is dissolved from the heat, the sucrose is then divided into fructose and glucose. Fructose gives the dish the overall sweetness since fructose is the sweetest sugar. The glucose from the white sugar, along with the glucose from the broken down lactose and brown sugar, form long chains when heated in the milk because they absorb and expand eventually separating into a loose liquid colloid (1). Once cooled they fold together (align) once more to form a more solid gel. Too much sugar in the recipe would inhibit the production of the starch granules since it is a structure weakener. More sugar in the recipe means the slowing of gelatinization because it competes with the cornstarch molecules for the absorption of water.
Brown Sugar
The brown sugar is added at the same time as the white sugar so they melt at the same time. Many traditional Natilla Colombianas have panela as an ingredient. Panela is a dark molasses-like sugar that is a by-product of sugarcane processing. However, it can be difficult to find. Brown sugar makes a good substitute because it is white sugar crystals coated with a layer of dark syrup or molasses, making them soft and clingy with a similar flavor profile. These crystals are hygroscopic when compared to white sugar (solely sucrose molecules) bringing moisture to the product when added to the milk. Brown sugar is also acidic because of the presence of acetic acid in the molasses. The small amount of acid helps to break down the proteins in the milk to enable a tertiary structure and create a three-dimensional network that is like a solid but mostly liquid.
Cloves
Originating from the Myrtaceae family, they are native to the Maluku islands in Indonesia. The cloves serve as an underlying flavor profile in this recipe. The intense aromatic spice is infused into the milk to create a subtly sweet flavor that pairs well with other slightly sweet spices such as cinnamon, nutmeg, and allspice. It is added after you pour the milk into the pot so it can boil with the milk and can have time to infuse.
Cinnamon Sticks
Cinnamon serves as the main flavor profile in the recipe. It provides a rich, spicy flavor and aroma to dishes while also providing many health benefits. They are best used in simmered beverages or slow-cooked stews to bring a proper infusion of warmth to the dish. This cannot be done with cinnamon powder as it is hydrophobic and releases flavor much quicker than cinnamon sticks.
Shredded Coconut
The shredded coconut is an addition of personal preference. It is added before the cornstarch and after the milk is boiled with the cinnamon and sugar so the flavor is incorporated but they do not cook. Furthermore, once the cornstarch is added, the gelling process begins making it difficult to add anything else. It also adds more body to the dish by creating a bond with the other molecules in the natilla. If coconut isn’t in your palette, then you can opt for raisins or even nuts.
Cornstarch
The cornstarch is added last when cooking since it is the thickener. The other ingredients need to be well incorporated before introducing the cornstarch slurry because once integrated, nothing else can be added. The gelling process is beginning.
2

When atoms with different attractions for electrons become bonded to each another, hydrogen bonding occurs. This process causes some of the atoms (i.e., nitrogen or oxygen) to become slightly negative in charge because they gain electrons. Some atoms (i.e., hydrogen) gain a slightly positive charge because they “lose” some of the shared electrons. This in turn creates a chain of events for the same thing to occur with other atoms. The negative and positive portions of the atoms become attracted to and attract other nearby molecules or even the same one. This creates a huge tangle of hydrogen-bonded strands because of the long glucose polymers. Thickeners are essential to any cook. The ability to thicken something comes from the incorporation of a complex carbohydrate and/or protein that binds to water. Water (H2O) has a huge presence in many foods and cooking processes and has a huge potential for hydrogen bonding. Cornstarch is the main ingredient used as the thickener while milk is the main liquid used. Since milk has 87-88% water content, this allows for greater hydrogen bonding and creates a perfect environment for the cornstarch to work.
amylose 3
amylopectin 4
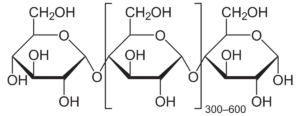
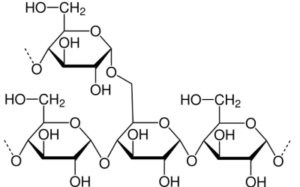
Starches are made up of two compounds – amylose and amylopectin – that determine the thickening power of the starch. Amylopectin is the compound that absorbs water and creates gelatinization. 75% is found in cornstarch, explaining its high power for thickening. After gelatinization, gelation occurs; amylose leaves the starch molecule into the surrounding water. The temperature at which gelation occurs is in the 200-220°F range which in turn requires simmering or boiling liquids for it to set once cooled. However, when using the cornstarch in the middle of cooking a slurry needs to be made. This means mixing the cornstarch first with a cold liquid using equal parts (i.e., water, milk, stock, or other liquid used in the recipe). This step is required since it ensures that all the cornstarch is suspended and is allowed to be distributed evenly throughout the rest of the mixture so the starch molecules can properly gelatinize. The direct addition of cornstarch into a hot substance can cause clumps of starch and grainy pockets.
To optimally activate the thickening power of cornstarch, it must be heated to a certain temperature; specifically, 95°C (203°F). Colombian Natilla requires you to make a cornstarch slurry because you are adding cornstarch to hot milk. Creating this slurry allows for even distribution of the starch molecules and prevents them from clumping in the milk. The amylopectin creates the perfect tool in the natilla to create that beautiful set and sheen appearance. When added to a recipe, cornstarch molecules absorb the water that is present. Heating these molecules causes them to swell and absorb a greater amount of water. Thoroughly cooking will cause the starch mix to expand by six to ten times its original size. The molecules will then set when the mixture is cooled. The cooling process (retogredation) further solidifies the food. However, there is one thing to be aware of. Heating the cornstarch mixture to or past the boiling point causes the molecules to deflate, creating a runny mixture. The molecules have a limited expansion. The cornstarch is added and only mixed on the heat until the mixture is just thickened enough to see the bottom of the pan. If left on the heat, the cornstarch molecules will deflate and the Colombian natilla will not have a thick consistency and will not set as needed.
Cinnamon Powder
This fragrant powder is only used in this dish as decoration. It adds a kick of cinnamon to enhance the taste of the natilla while pairing with the nutmeg, coconut flakes, and cinnamon stick infusion.
Substitution
My goal is to eat more plant-based, so, in my recipe, I will change the type of milk used. Instead of cow’s milk, I will be using coconut milk. This will create a stronger coconut flavor. As explained above, cornstarch molecules absorb water and expand to gelatinize. Cow’s milk is made up of 88 – 87% water while coconut milk is about 50% water – the rest being fats and proteins. The water content in coconut milk will still allow for the cornstarch to do its thickening job.
Looking to make a vegetarian/vegan alternative to traditional Natilla Colombiana? Click here!
———————————————————————————————————————————————————
References:
Baking with cornstarch: Bob’s Red Mill. https://www.bobsredmill.com/blog/healthy-living/baking-with-cornstarch-everything-you-need-to-know/ (accessed May 5, 2022).
McCormick Kitchens. Flavor story: Cinnamon Sticks. https://www.mccormick.com/articles/mccormick/flavor-story-cinnamon-sticks (accessed May 5, 2022).
McCormick Science Institute. Cloves. https://www.mccormickscienceinstitute.com/resources/culinary-spices/herbs-spices/cloves (accessed May 5, 2022).
Potter, J. Cooking for geeks: Real science, great cooks, and good food; O’Reilly: Beijing, 2016; pp 408–410.
A. Wallert, M.; J. Provost, J.; L. Colabroy, K.; S. Kelly, B. The Science of Cooking : Understanding the Biology and Chemistry Behind Food and Cooking, 1st ed.; John Wiley & Sons, Incorporated, 2016; pp 18-20. Retrieved from ProQuest Ebook Editor https://ebookcentral.proquest.com/lib/dickinson/reader.action?docID=4530809&ppg=80 (accessed Apr 16, 2022).
Photo References:
Chistruga, D., 2021. Ingredients for Colombian Christmas Custard (Natilla Colombiana). [image] Available at: <https://www.thespruceeats.com/natilla-colombian-christmas-custard-3029402> [Accessed 5 May 2022].
(1) Wikipedia. sucrose structure; 2022. https://en.wikipedia.org/wiki/Sucrose
(2) Anatomy and Physiology. hydrogen bond between water molecules; 2013. https://openstax.org/books/anatomy-and-physiology/pages/2-2-chemical-bonds?query=hydrogen%20bond&target=%7B%22type%22%3A%22search%22%2C%22index%22%3A0%7D#fs-id1493624
(3) Wikipedia. amylose structure; 2022. https://en.wikipedia.org/wiki/Amylose
(4) pngwing. amylopectin structure. https://www.pngwing.com/en/free-png-yckkm
Recipe Reference:
Diane. Colombian Natilla. Sweet y Salado, December 10, 2012. https://www.sweetysalado.com/en/2012/12/colombian-natilla.html (accessed 2022-05-05).