Summary of:
Dirren, E., Aebischer, J., Rochat, C., Towne, C., Schneider, B. L., & Aebischer, P. (2015). SOD1 silencing in motoneurons or glia rescues neuromuscular function in ALS mice. Annals of Clinical and Translational Neurology.
Brandon Goldson and Jessica Snyder
What is ALS?:
Also known as Lou Gehrig’s disease, ALS (Amyotrophic lateral sclerosis ) is a fatal and incurable disorder primarily characterized by rapid progressive degeneration of motor neurons in the brain and spinal cord. This degeneration of the motor neurons leads to immobility, paralysis and eventually death 3-5yrs after diagnosis. About 5,000 people are diagnosed with an estimated 30,000 people living with ALS in the United States each year. Though ALS occurs in the world with no racial or socioeconomic boundaries, risk factors include age, sex and genetics. Regarding genetics, 10% of the people suffering from ALS display an inherited form of the disease. Within that population, 20% present a mutation in the gene encoding superoxide dismutase 1(SOD1).
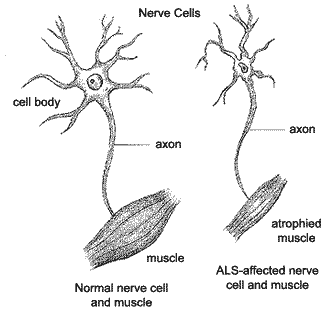
What is the Known Pathophysiology of ALS and SOD1?:
Over 160 mutations of SOD1 have been found to cause ALS. These mutations cause an increase in toxicity and a degeneration of motor neurons in the brain and spinal cord.
What is the Hypothesis?
MiR Sod1 and MiR -b SOD1 effectively silence SOD1 (g93A) activity in mice expressing the human mutated gene. This silencing improves function and delays degeneration and symptoms associated with ALS in mice.
What is the Experimental Design
The effect of a miRNA designed to silence the SOD1 gene was tested both in cell culture and a mice model. Both models contained a SOD1 gene mutation. After the silencing effect was confirmed in vitro, the researchers tested the efficacy of two treatments in SOD1 transgenic mice. The first treatment was AAV6:miR SOD1 and the second was a combination of AAV6:miR SOD1 and AAV9:miR SOD1. In all but the final experiment, the mice were given the treatment soon after birth.
The effect of the treatment in the mouse model was tested in the following ways:
- examine whether or not the AAV is localized in correct cells (motor neurons and astrocytes are test in the spinal cord)
- Observe SOD1 expression with and without the AAV miR treatment
- motor function test of mice with & without treatment (swimming latency test and rotarod test)
- test of proteins contained within muscle sample
- staining of spinal tissue to see motor neurons status (intact or not) as well as the viability of neuromuscular junctions
- muscle fiber thickness to evaluate whether or not muscle atrophy was lessened
- Finally, adult transgenic SOD1 mice were given the treatment and evaluated for viability, motor function and the state of neuronal cells in the spinal cord.
What are the Key Results?:
1: Suppressing of SOD1 in astrocytes and motor neurons : Figure 1
- Quantification of human SOD1 levels relative to miR control confirms that human SOD1 expression is significantly reduced in cells overexpressing miR SOD1 and miR-b SOD1.
2: Delays Disease Progression and Improved Disease Outcome in the following ways
- Increase in intact spinal motor neurons: Figure 4
- NMJ function was rescued to 90% as compared to control G93ASOD1 mic
- Rescues Neuromuscular junctions: Figure 4
- As compared to WT and PBS-injected G93ASOD1 mice, significant preservation of motoneuron integrity at all levels of the spinal cord was observed in AAV6:miR SOD1 and AAV6 + AAV9:miR SOD1 groups.
- Increase in muscle fiber diameter (rescues muscle atrophy): Figure 5
- Hematoxylin and eosin staining of gastrocnemius muscle sections displayed maintenance of muscle integrity with respect to AAV9:miR SOD1 mice compared to control transgenic mice.
What is the Broader context?
These results show promise for the treatment of familial ALS. The maintenance of motor neurons and NMJ is key to attenuating disease symptoms and improving quality of life in ALS patients. Full or partial rescue of neuromuscular function following neonatal and adult ICV infection of AAV-miR SODI may establish the therapeutic silencing approach as an effective treatment for ALS. Furthermore, this paper confirms that SOD1 silencing in specific cell types such as motor neurons and astrocytes is crucial to this therapeutic targeting. Additionally AAV based therapeutic targeting may be used to alter other genes related to ALS prevalence.
P.S. Helpful YouTube Videos about the Nervous System Below!
I find this article and your summary fascinating, especially in the context of different types of cells affected by ALS. I am interested in how the researchers tested the delivery of SOD1 to motor-neurons or astrocytes, and found an improvement in the disease outcome. I am also curious as to how the treatment differs in the two cell types, specifically since the suppression of SOD1 preserved innervation while the motor-neurons were only partially projected.
My spouse also used the www multivitamincare .org ALS, amyotrophic lateral sclerosisorganic herbal supplement ,2 times daily. The treatments are incredible and we have told so many people about it.
I am curious about the animal model used for this study. In human patients, 160 different mutations in the SOD1 gene have been identified. Do all of these mutations have essentially the same phenotype? If not, how does the phenotype of the transgenic model model compare? It would be interesting to understand more about the specific toxic effects of the various mutations in both humans and animal models.
This article shows interesting promise for the treatment of one form of ALS. However, it was pretty unsatisfying to read that this paper focuses on the mutation in the gene encoding SOD1, which only accounts for 2% of ALS occurrence. Obviously, connections can be made to relate these findings to other forms of ALS, but this statistic really surprised me in showing just how much is unknown about ALS.
Although the paper illustrates some interesting and conclusive results, I am not convinced as to how this would work as a treatment in humans. The authors state that the mice experienced various side-effects (with unknown causes) such as ataxia, infections, and sudden death. Additionally, when the authors discuss the effects on muscle cells, they mention that some mice actually have increased muscle size. Is it possible that there are steroidal implications with this treatment?
While the researchers on this project were successful in their analysis of SOD1-related cases of ALS, due to the fact that these mutations only comprise 2% of all ALS cases, it begs the question as to whether or not these findings translate to larger pool.
Their AAV treatment seems to be an effective treatment, but I wonder if it can be applied to other genes, like C9orf72, which cause a large number of the familial ALS cases.
Also, I’m curious if any of their findings help shed light on the processes which cause ALS that are not genetic as this comprises nearly 90% of all cases.
In the discussion, the authors reiterate key differences in the neuroprotective effects between the two vectors at the lumbar level following IT injection of adult mice. They found that SOD1 silencing in motoneurons was more neuroprotective than astrocyte-specific SOD1 silencing. What is thought to account for this discrepancy?
I found it interesting that the results of this article showed that silencing SOD1 had such a large impact on the prevention of the disease. However, I am unsure how easy treatment in humans would be. Would injection into the spinal cord be necessary or could a drug be developed? In addition, what effect does silencing SOD1 have on other cells in the body besides motoneurons and astrocytes?
I think it is interesting that several of our papers this year have utilized miRNA silencing! As far as this paper goes, one thing that I wish they had discussed a little more were the side effects seen in some miR SOD1 mice. I am curious as to whether these complications were due to a downstream or unintended effect of the miRNA, or if there was some sort of inflammatory damage caused by the genotype or presence of a viral vector, etc. In their conclusion, the authors suggest another possibility, that the effects seen are caused by a genetic defect that this genotype of mouse does not typically survive to experience. However, I feel that this is an area that deserves further investigation, particularly if the knockdown of SOD1 or an associated protein is the cause.
This paper gives a glimpse at how complex ALS is as a disorder. While the SOD1 mutation only accounts for 2% of the known cases, I think through investigating its function and the cell type that benefits most from therapeutics, there are gains made through their findings. I find their use of AAV9-shRNA as a therapeutic treatment to be fascinating, and wonder how exactly this works in the process of targeting specific locations. Along those lines, I would be interested to see this move into human treatments. While this research is certainly helpful in coming closer to a therapeutic regiment for certain patients, I am not convinced that it is the most worthwhile ALS research in terms of benefiting a large number of patients diagnosed with ALS.
Nice comments, everyone!
I am unsatisfied with the background information on SOD1 in the introduction section of this paper. All it states is that mutations in SOD1 result in gain of toxicity and that mutant SOD1 “can perturb a number of cellular functions” with only 1 reference to this statement. This language is too vague. What is the molecular mechanism responsible for the effects of SOD1 mutation?
This paper gives really clear immunostaining that I could see what is going on with their immunostaining. I like their figures. I like how the paper goes further with injecting SOD1 (therapies). However, I was confused how they can suggest there was improved muscle response in treated animals compared to PBS-injected mice by looking at figure 6B. Instead of analyzing what they had from data, in the result section, I feel like they should just state what they had especially in figure 6D. and I wish they could just mention or label where they got the information on NMJs (from Figure 6H) in the result section. In addition, wouldn’t figure 5 is supporting ideas of the paper have rather than the main key figure?
I would like to further understand the effects of SOD1 in all forms of ALS. If it is known that with the isolation of SOD1 mutations there is an increased toxicity and degeneration of motor neurons in the CNS how can researchers relate this to those other 90% without a hereditary version of ALS. Could age and unknown exposure to environmental cause mutations in the gene or the way in which it is replicated? I am very curious to further expand upon the role of SOD1 in those ALS cases that have not been deemed hereditary. Along with any findings on this could treatment or therapy be overlapped? Or could we gain a better detection or prevention method of this degeneration and toxicity among motor neurons in the CNS.
We all did our best to seek help for this disease, no medications they prescribed worked ,we were all scared we might lose him due to his condition, as he had been his brother’s caregiver a few years earlier for the same disease before he passed. doctor recommend natural treatment from multivitamin herbal cure for his ALS we have no choice to give a try on natural organic treatment ,this herbal cure has effectively reverse my father condition ,losing his balance which led to stumbling and falling stop after the completing the herbal supplement which include his weakness in his right arm and his speech, home remedies from multivitamincare. org is the best although their service is a little bit expensive but it worth it, they save lives.
I was diagnosed in March 2017 but was running around from doctor to doctor before I finally get a result that I was free from MND ALS. Mine started on top and progressed to the bottom I could walk very little but needed assistance as I have no balance. It is sad all the time that we thought this disease has no cure with all the technology we have while there are some formulas there that can relieve all symptoms and get rid of this of Amyotrophic lateral sclerosis (ALS). I’m passing this info to anyone at there because www multivitamincare org has the right cure and caregiver for this disease ….I took various supplements, medicine prescribed by a neurologist, massage, and physiotherapy still the disease was progressing very fast until the ALS formula from that company.
Hope this is allowed on here if not I understand. Around age 60 I noticed that my handwriting was getting smaller and I was writing faster. I also noticed twitches on my right hand. The doctor went over my different symptoms and he suspected I’d either had a small stroke or the beginnings of Parkinson’s. After finding a neurologist and some testing I was diagnosed with Early stages of Lou Gehrig’s disease. That was 2 years ago. I took Radicava four times a day to control my symptoms, which include drooling, muscle weakness, gait problems, swallowing difficulties, and my while body feeling faint, A year ago, I began to do a lot of research and came across Health Herbs Clinic (ww w. healthherbsclinic. c om) and their ALS/MND HERBAL TREATMENT. After seeing positive reviews from other patients, I quickly decided to get started on the treatment, ( NOTE: Our family doctor was informing my family to prepare for my funeral as I had a year to live at that point ), After 2 months I experienced significant reduction/decline in major symptoms, including muscle weakness, speech problems, difficulty swallowing, fatigue and others, The truth is you can get off the drugs and help yourself by trying natural methods, i live symptoms free. I’m forever thankful to nature and Health Herbs Clinic
I’m living proof that you can live comfortably with COPD. I have emphysema, but emphysema doesn’t have me. After years of living with chronic obstructive pulmonary disease (COPD) with a bronchodilator medication approach, I didn’t have much improvement till I started using World Rehabilitate Clinic Herbs Formula which has made a tremendous difference With a period of 2 months. They specialize in internal and pulmonary medicine, all my symptoms have declined completely, I feel normal again, but I have a few weeks left completing the treatment. I can now go about my daily activities. It’s also crucial to learn as much as you can about your diagnosis. Seek options visit ( worldrehabilitateclinic. com).
I am amazed by Dr Timothy herbal products. I Have had Genital herpes for over 4 years with frequent outbreaks. Sometimes average 2-3 times per month. Before one breakout could end, the next would begin. Nothing has helped me. I came online in search for a possible way to see how I can fight this virus so I found Dr Timothy here online after seeing a lot of testimonies of how he cured people from herpes and other diseases with natural herbs so I decided to give it a try, I only took his herbal product for good two weeks and I was completely cured. I recommend Dr Timothy remedy to anyone suffering from herpes that wants to be completely cured too. contact website https://5dc41d5b6d60d.site123.me or email goodhealthherbalfoundation1 @ gmail. com for quick response.
Hello
Do you need hacking services or faced with delays and unnecessary excuses from fake hackers on your job? Worry no more,we are here for you,we will give you the best result without stress.We are professional hackers, what hacking services do you need? We will render it with swift response and no delay, your job is 100% guaranteed.Contact us at incrediblehacker247@yahoo.com for more information.Our services includes:
*School grade hack,
*hack into email accounts,
*all social media accounts,
*school database to clear or change grades,
*Retrieval of lost documents
*Erase criminal records
*Company records and systems,
*Bank accounts,
*Iphone Hack
* Credit card hacking,
*Website crashed hack
* Credit score hack,
* Recover stolen Bitcoin or crypto money
* Monitor your partners phone, etc.. Contact us now any kind of hack job.
A lot of people believe there is no cure for ALS disease, which is not true. Everyone has to understand that natural treatment is the best among all. My Dad is 100% fine after using Dr Agumba ALS herbal product. I’m so happy that he got healed and I do believe in natural herbs right from the beginning especially when you got it from a good and understanding naturopathic. It’s better and more active for getting rid of every disease. Anyone who needs ALS herbal cure, here is Dr Agumba Email dragumbasolutioncenter@gmail.com
I have been suffering from Herpes for the past 1 years and 8 months, and ever since then I have been taking series of treatment but there was no improvement until I came across testimonies of Dr. Silver on how he has been curing different people from different diseases all over the world, then I contacted him as well. After our conversation he sent me the medicine which I took according .to his instructions. When I was done taking the herbal medicine I went for a medical checkup and to my greatest surprise I was cured from Herpes. My heart is so filled with joy. If you are suffering from Herpes or any other disease you can contact Dr. Silver today on this Email address: drsilverhealingtemple@gmail.com or Whatsapp +2348120513902
OPTIMISTIC HACKER GAIUS: IS THE BEST COMPANY FOR CRYPTO CURRENCY RECOVERY AND HOW TO RECOVER LOST CRYPTO FUNDS
The investment scheme initially drew me in with the promise of large profits and the guarantee of a safe investment. But as time passed, I came to see that the investment was a complete fraud. I was left feeling dissatisfied and powerless after losing access to my money. It was a terrible experience, and I thought I was out of choices. I looked up a few cryptocurrency recovery businesses online and read customer endorsements and reviews after doing some investigation. To assist me in getting my investment back, I had to discover a reliable provider. I gave it some thought before deciding to get in touch with OPTIMISTIC HACKER GAIUS who received great feedback and endorsements. Their professionalism and promptness really pleased me, and they offered me hope by thoroughly explaining the recuperation process. After he recovered well, I agreed to review the fantastic work he did. Optimistic Hacker Gaius is a great resource for any fraud victim looking for assistance in recovering their lost cryptocurrency.
Contact details:
email… Optimistichackergaius @ seznam. cz
Call or use WhatsApp at +1..6..0…1..4..6..0..9…4..7..7.
the website page is; https://optimistichackegaius.com