The second task in our investigation was to adjust the amount of baking soda in our XXL Sugar Cookie recipe.
You take the giant sugar cookie out of the oven and notice it’s soft and puffy like a cloud. There’s some color around the edges but the colorful sprinkles pop out against the mainly pale surface. You’re mesmerized by how delicious this cookie looks and can’t wait to devour it piece by piece. But why is this cookie the way it is? The answer is leavening agents, specifically baking soda.
Leavening agents affect many aspects of baked cookies. Using the recipe you can find here, we wanted to investigate how the texture, appearance, and taste of the cookie would change if we added more or less baking soda. The recipe originally calls for ¼ tsp of baking soda and ¼ tsp of cream of tartar. If we doubled the amount of baking soda for one batch (½ tsp baking soda) and then halved the amount of baking soda for another batch (⅛ baking soda), in what ways would the cookie change?
To understand more about the science behind this recipe, visit our Blossom Recipe.
Science behind baking soda
Both baking soda and cream of tartar are used in this recipe. What you may or may not know is that baking soda, also known as sodium bicarbonate, is a base which means it has a pH of above 7. Cream of tartar on the other hand is an acid, and so has a pH of below 7 (Potter, 2015). When these two ingredients are mixed together with a liquid, the pH of both substances neutralize. This chemical reaction produces carbon dioxide which creates air pockets in the cookie dough and increases the volume. Due to the carbon dioxide, the cookie rises and becomes puffy when baked (Swartz, 2017). Below is a picture of the chemical reaction that occurs between baking soda and cream of tartar.
We already know the combination of baking soda and cream of tartar produces carbon dioxide, but let’s take a look on an atomic level. When a base and an acid mix, there’s a proton exchange. Bases are proton “stealers” while acids are proton “givers”, and when they exchange protons, carbon dioxide is released (Potter, 2015).
Alterations
Now that you know why baking soda and cream of tartar are important for this recipe, think about whether the measurements really matter. Is more baking soda better? Is less of it better? I made three batches of this recipe, one following the original recipe, one with ½ of baking soda (⅛ baking soda), and one with two times as much baking soda (½ baking soda).
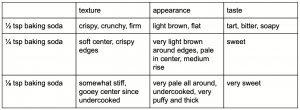
Here are the results:
Doubled

When I doubled the amount of baking soda, several things happened. First, the texture was not as soft as the original cookie but rather crispy and firm. It was also very flat. What happened was that too much carbon dioxide was produced. The air pockets formed by the carbon dioxide combined and eventually rose to the top of the cookie (Davison, 2012). All the gas that was produced was then released because the air pockets were popped (think of a balloon and what happens when too much air is pumped into it). The result was a flat and dense cookie.
The second observation I made was that the color of the cookie was a lot darker than the original one. This is due to the Maillard reaction which is a reaction between an amino acid and sugar (Provost, 2016). Amino acids have an acidic end and an alkaline end. It’s the alkaline part of the molecule that reacts with sugar and then results in browning. Since baking soda is an alkaline, an excess amount in the recipe creates an alkaline environment which then speeds up the Maillard reaction (Davison, 2012). Another way to look at this is in terms of pH. The addition of baking soda increases the pH of the recipe which means a “proton is removed from the amino group and [so then] the reaction can occur” (Provost, 2016).
Lastly, the taste of the cookie was not very pleasant. When the right amount of baking soda and cream of tartar react, they neutralize and reduce the flavor of one another. However, when there is not enough acid to do this, the soapy taste of the excess baking soda is left over (Davison, 2012).
Normal

With no changes to the amount of baking soda in the recipe, the cookie was soft, had a pale center with light brown edges, and didn’t taste too sweet. There was just the right amount of carbon dioxide to give the cookie a nice puffy look and soft texture. Also, the Maillard reaction was not sped up, but did have some effect on the edges. Lastly, the baking soda and cream of tartar neutralized so that the soapy taste of sodium bicarbonate was not left behind.
Halved

When I halved the amount of baking soda, I noticed the center of the cookie was undercooked. Baking soda is meant to slow down protein coagulation (transition of a liquid state into a solid state) which is supposed to give the cookie “more time to spread before it sets” (Swartz, 2017). However, protein coagulation was not slowed down and so the cookie did not spread like it was supposed to. The center remained bulky and so the heat was not able to cook it fast enough. Carryover cooking wasn’t able to cook the center either because it was too thick. This explains why the cookie looked puffy. It was because it didn’t spread, not because of carbon dioxide.
I also noticed that the color was very pale. The reduction of baking soda made the cookie more acidic. This prevented the Maillard reaction from happening because the amino group that was supposed to react with sugar “becomes a protonated ammonium group, which is very unreactive toward” sugar (Provost, 2016).
Additionally, this cookie was very sweet. Baking soda is supposed to reduce the acidity of a recipe by increasing the pH. However, since cream of tartar doesn’t have a very strong taste, less baking soda didn’t result in a more acidic tasting cookie, but a sweeter one.
Tip
If the sugar cookie ends up looking extremely flat, but you used the correct amount of baking soda and cream of tartar, it might be because the baking soda has expired. Try adding some baking soda and cream of tartar to a small bowl of water. If bubbles begin to form, then the baking soda is good.
To learn more about how egg alterations affect this recipe, click here.